Iveric Bio Announces Vision Loss Reduction Data in Geographic Atrophy from Avacincaptad Pegol GATHER Trials
- Post-hoc time-to-event analysis signals up to 59% risk reduction in rate of vision loss compared to sham treatment at 12 months -
- Analysis to be presented at the ARVO Annual Meeting from
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230228006487/en/
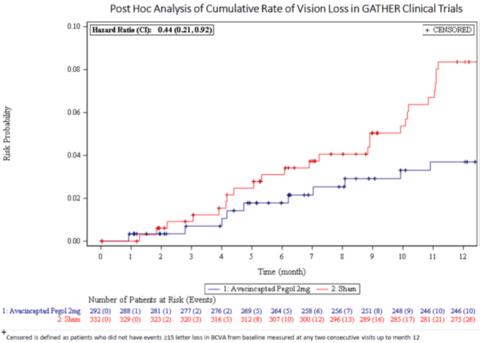
Post-Hoc Analysis of Cumulative Rate of Vision in GATHER Clinical Trials (Photo: Business Wire)
Results were consistent in the GATHER1 and GATHER2 clinical trials independently, signaling a 44% reduction (
“GA is a devastating disease, which can lead to vision loss and irreversible blindness taking away the patients’ ability to drive, read and see their loved ones,” says
About Avacincaptad Pegol
Avacincaptad pegol (ACP) is an investigational drug for treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD) that is currently under evaluation for safety and efficacy by the
About Geographic Atrophy
Age-related macular degeneration (AMD) is the major cause of moderate and severe loss of central vision in aging adults, affecting both eyes in the majority of patients. The macula is a small area in the central portion of the retina responsible for central vision. As AMD progresses, the loss of retinal cells and the underlying blood vessels in the macula results in marked thinning and/or atrophy of retinal tissue. Geographic atrophy, associated with AMD, leads to further irreversible loss of vision in these patients.
About the GATHER Clinical Trials
ACP met its primary endpoint in the completed GATHER1 clinical trial and the ongoing GATHER2 clinical trial, both of which are randomized, double-masked, sham-controlled, multicenter Phase 3 clinical trials. These clinical trials evaluated the safety and efficacy of monthly 2 mg intravitreal administration of ACP in patients with GA secondary to AMD. For the first 12 months in both trials, patients were randomized to receive either ACP 2 mg or sham monthly. There were 286 participants enrolled in GATHER1 and 448 participants enrolled in GATHER2. The primary efficacy endpoints in both pivotal studies were based on GA area measured by fundus autofluorescence at three time points: Baseline, Month 6, and Month 12. The mean rate of growth (slope) in GA area from baseline to month 12 using observed data was 35% in GATHER 1 and 18% in GATHER2. In GATHER1 and GATHER2 combined, the most frequently reported treatment emergent adverse events at 12 months in the 2 mg recommended dose were related to the injection procedure. The most common adverse reactions (≥ 5% and greater than sham) reported at 12 months in patients who received ACP 2 mg were conjunctival hemorrhage (13%), increased IOP (9%), and CNV (7%).
About
Forward-looking Statements
Any statements in this press release about the Company’s future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements include any statements about the Company’s strategy, future operations and future expectations and plans and prospects for the Company, and any other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “may”, “might,” “plan,” “predict,” “project,” “seek,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. In this press release, the Company’s forward-looking statements include statements about its expectations regarding the results and implications of the clinical data from its GATHER1 and GATHER2 trials of ACP in geographic atrophy, including the relevance of post-hoc analyses from these trials, and the potential safety and efficacy of ACP in treating GA. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to expectations for regulatory matters, interpretation of clinical trial results by the scientific and medical community, developments from the Company’s competitors and the marketplace for the Company’s products, and other factors discussed in the “Risk Factors” section contained in the quarterly and annual reports that the Company files with the
References
-
Lindblad AS, et al, and
AREDS Research Group . Arch Ophthalmol. 2009;127(9):1168-1174
View source version on businesswire.com: https://www.businesswire.com/news/home/20230228006487/en/
Investor:
Senior Vice President, Investor Relations
kathy.galante@ivericbio.com
or
Media:
Senior Director,
jeannie.neufeld@ivericbio.com
Source:
